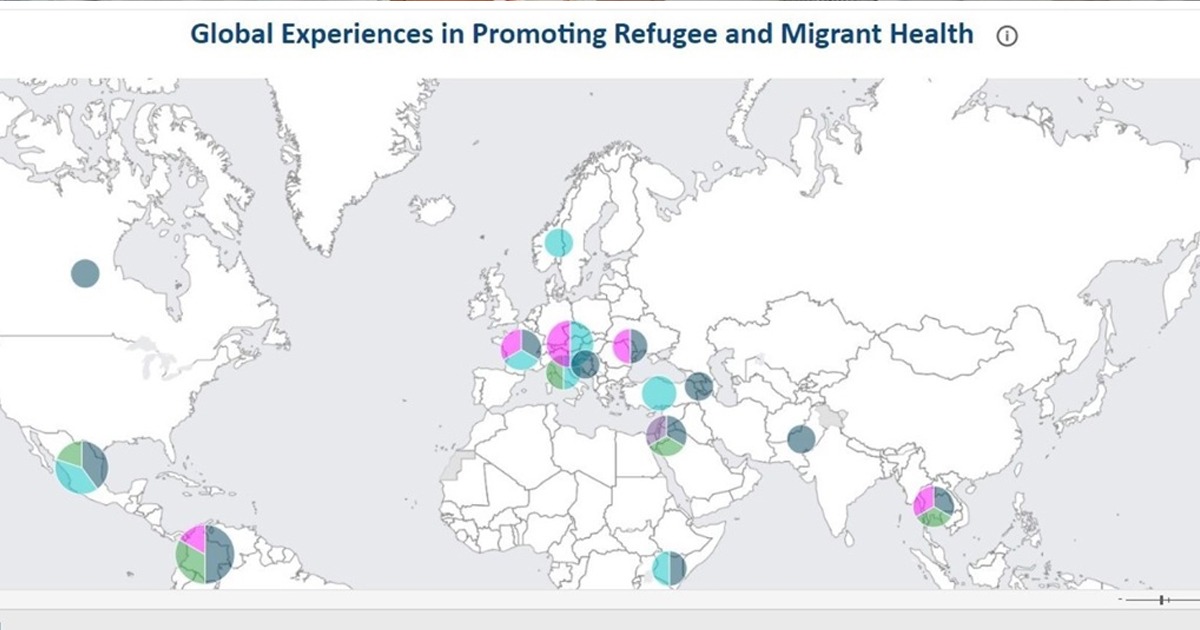
Decentralized clinical trials offer great benefits as they promote more diverse research, reduce costs, and also avoid risks of exposure to diseases such as COVID-19.
Due to the COVID-19 pandemic, the application of clinical trials was considerably reduced, therefore the decentralized clinical trial modality was the most appropriate to continue these activities during the health emergency.
Clinical trials are one of the most reliable methods to generate scientific evidence in new therapeutic approaches: However, when these trials must be carried out on a large scale, it is necessary for the participants to go to a data collection center with the specialists. This generates operational and time costs for both parties, participants and researchers.
A large part of the clinical trials do not reach their enrollment goals in a timely manner, and in addition the pandemic has further restricted this type of method. Therefore, the best option to improve enrollment rates in clinical trials, as well as reduce costs, the most viable option is decentralized clinical trials.

“Decentralized trials generally refer to trials in which recruitment and data collection procedures are not restricted to fixed locations. Participants can perform the testing activities where they are, including at home, using remote technologies, potentially heightening engagement and enhancing the study participants' journey," explain the authors of the Pandemic-Proof Recruitment and Participation study at a fully decentralized trial in patients with atrial fibrillation (DeTAP) published in npj Digital Medicine.
Unlike traditional trials, new decentralized trial protocols can speed up recruitment of participants. Also, thanks to new technologies it is possible to carry out remote studies. “Rapidly emerging digital health technologies can help remotely obtain the objective physiological data that cardiovascular trials often require,” the study explains.
The aforementioned study sought to validate the feasibility of a completely virtual decentralized clinical trial for cardiovascular interventions. Since no trial of this type, in phase 3 and on a large scale, has used a completely decentralized protocol and that includes physiological measurements obtained remotely.
Traditional recruitment for that study yielded only six participants, as opposed to the more than 400 participants recruited through social media tools. In 12 days, recruitment through social media advertising managed to enroll 94 participants and more than 300 eligible patients on the waiting list.
Ultimately, the study succeeded in reporting the feasibility of fully decentralized and potentially scalable cardiovascular intervention trials that included remote physiological monitoring among patients with atrial fibrillation, with strong virtual recruitment, strong commitment, and successful monitoring of oral anticoagulation adherence.
In this way, the study presents a basis that may be useful for the development of future protocols to carry out decentralized trials specialized in cardiovascular monitoring.
Learn more at: https://www.nature.com/articles/s41746-022-00622-9