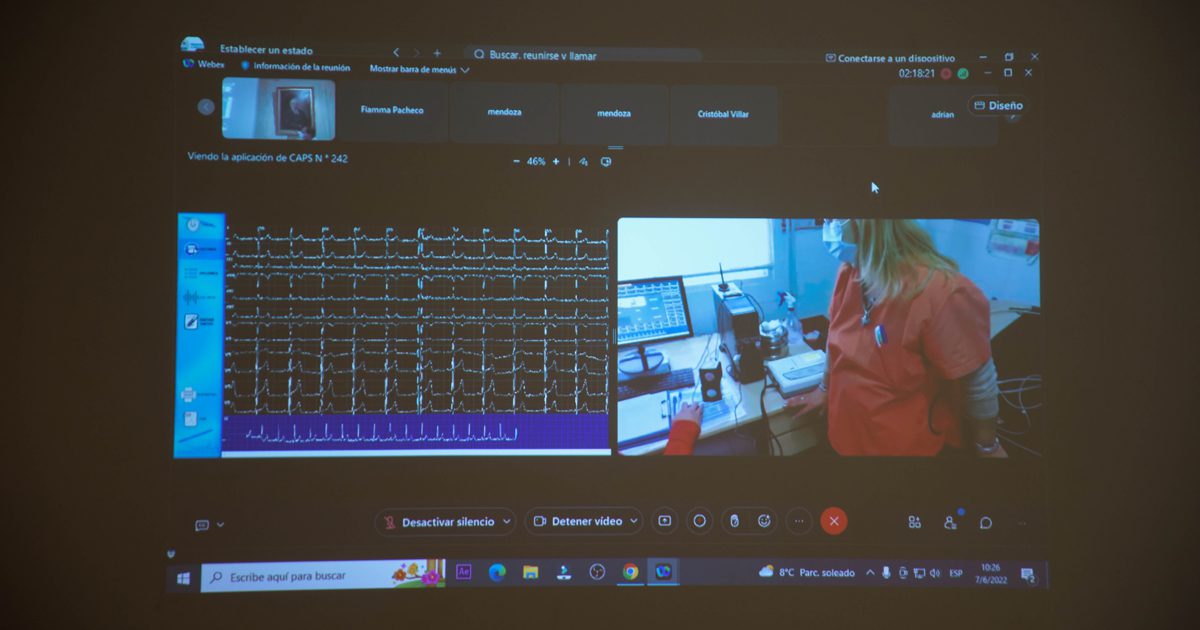
The Food and Drugs Administration (FDA), published a guidance about enforcement policies for non-invasive remote monitoring devices used to support patient monitoring during the coronavirus disease-2019 (COVID-19), public health emergency.
The FDA made this guidance to provide a policy to help expand the availability and capability of non-invasive remote monitoring devices, namely telemedicine devices. Devices that supports patient monitoring while reducing patient contact and exposure to COVID-19.
This policy will only be available during the public health emergency derived by COVID-19 pandemic. Due to the health emergency, the guide has been implemented immediately without any changes or comments from the public.
The FDA is allowing that the hardware used for remote monitoring will be commercially available, even with additional information about COVID-19. This represents a perfect telemedicine tool in times of health crisis.
In addition, modification of hardware to cover specific needs, such as adding Bluetooth or other ways for connecting devices, is considered.

“Allowing these devices to be used remotely can help health care providers access information about a patient’s vital signs while the patient is at home, reducing the need for hospital visits and minimizing the risk of exposure to coronavirus,” said FDA Principal Deputy Commissioner Dr. Abernethy, when referring to the steps taken by the FDA.