1. How many COVID-19 vaccines are available?
Vaccine development generally take years of research and testing before they reach the public, but in 2020 and in the COVID-19 pandemic context, scientists around the world are working to produce safe and effective vaccines against SARS-CoV-2 in record time. There are currently hundreds of research groups developing and testing different types of vaccines. As of January 2021, 66 vaccines are already in human clinical trials, and 20 have reached the final stages of testing.
The most advanced vaccines have already been approved for emergency use by several countries. Those include:
- abdullah
- AstraZeneca/Oxford
- CanSinoBIO
- Covaxine
- Johnson & Johnson
- modern
- Novavax
- Pfizer/BioNTech*
- Synopharm
- Sinovac
- Sputnik V
*On August 23, 2021, the United States Food and Drug Administration (FDA) granted full approval to the Pfizer/BioNTech vaccine for use in people 16 years of age and older.
*On January 31, 2022, the FDA granted full approval to the Moderna vaccine for use in people over 18 years of age.
The vaccines that have been approved by COFEPRIS (Federal Commission for the Protection against Sanitary Risk) for use during the health emergency in Mexico are:
- abdullah (29/12/2021)
- AstraZeneca/Oxford (04/01/2021)
- CanSinoBIO (08/02/2021)
- Covaxine (06/04/2021)
- Johnson & Johnson (27/05/2021)
- modern (17/08/21)
- *Pfizer/BioNTech (11/12/2020)
- Synopharm (25/08/21)
- Sinovac (09/02/2021)
- Sputnik V (09/02/2021)
https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html
https://www.gob.mx/cofepris/acciones-y-programas/vacunas-covid-19-autorizadas
* On March 3, 2022, COFEPRIS authorized the application of the Pfizer/BioNTech vaccine for children between 5 and 11 years of age
2. What are the ingredients of the COVID vaccines?
Each vaccine has a formulation which generally consists of the antigen or protein of the SARS-CoV-2 virus which will be responsible for triggering the immune response (defense mechanism) of the organism and against which the antibodies and activated cells are produced. In addition, necessary substances are included to maintain the stability of the antigen before and after the application, as well as to stimulate the response capacity of the immune system after the application of the vaccine.
The ingredients of the three vaccines currently available for application are described below:
Pfizer/BioNTech's COVID-19 vaccine contains the following ingredients:
- Messenger ribonucleic acid (mRNA)Is the only active ingredient in the vaccine. The mRNA molecules contain the genetic material that provide instructions for our body on how to make a viral protein that triggers an immune response within our bodies. spike or spike that will trigger the immune response.
- Lipids: Their main role is to protect the mRNA and provide somewhat of a “greasy” exterior that helps the mRNA slide inside the cells.
- Salts : The following salts are included in the Pfizer vaccine and help balance the acidity in your body.
- Sugar (sucrose): This ingredient helps the molecules maintain their shape during freezing.
Moderna COVID-19 Vaccine contains the following ingredients:
- mRNA: Like the Pfizer BioNTech vaccine, Moderna’s also uses mRNA technology to build antibodies against COVID-19.
- Lipids: The Moderna vaccine also requires lipids to help deliver the mRNA to the cells.
- Stabilizing substances such as salts and sucrose.
AstraZeneca/Oxford vaccine is made up of:
- A viral vector called Adenovirus ChAdOx1, which is a chimpanzee adenovirus, does not cause disease in humans. This virus carries with it the genetic material (similar to the mRNA of the Pfizer and Moderna vaccines), which will be responsible for producing the SARS-CoV-2 spike protein.
- In addition to the vector of the genetic material, the vaccine includes substances to maintain its stability.
The Sputnik V vaccine, developed by the Gamaleya National Center, contains:
- Two viral vectors, Adenovirus 26 in the first dose and Adenovirus 5 in the second dose. Both viruses are weakened so as not to cause disease in people, but capable of carrying the genetic material of the virus, specifically the gene that carries the instructions for human cells to produce the SARS-CoV-2 spike protein.
- In addition to the vector of the genetic material, the vaccine includes substances to maintain its stability.
The CanSinoBio vaccine contains:
- A viral vector called Adenovirus 5, which is a weakened virus so as not to cause disease in people, but capable of carrying the genetic material of the virus, specifically the gene that carries the instructions for human cells to produce the SARS-CoV-2 spike protein.
- In addition to the vector of the genetic material, the vaccine includes substances to maintain its stability.
- This vaccine is given in a single dose.
The Johnson & Johnson contains:
- A viral vector called Adenovirus 26, which is a weakened virus, as in the CanSinoBio vaccine, so as not to cause disease in people, capable of carrying the genetic material of the virus, specifically the gene that carries the instructions for human cells to produce the spike protein of SARS-CoV-2.
- In addition to the vector of the genetic material, the vaccine includes substances to maintain its stability.
- This vaccine is given in a single dose.
The Novavax contains :
- Protein spike recombinant assembled into three-dimensional nanoparticles
- an adjuvant
The abdullah contains:
- Recombinant protein S (receptor binding domain), cloned into yeast and purified.
- Aluminum hydroxide as an adjuvant
- phosphate buffer.
COVID-19 vaccines Sinovac Biotech, Sinopharm and Covaxine, contain the SARS-CoV-2 virus inactivated by a chemical called beta-propiolactone that deprives the virus of the ability to reproduce (replicate) inside human cells, preventing it from causing disease. However, the spike protein spike remains intact and with all the ability to stimulate the body's immune response to generate protection. In addition to the inactivated virus, the vaccine contains an aluminum-based substance called an "adjuvant" that serves to further stimulate the immune system to respond appropriately to the vaccine.
Vaccine
Country
Dose
Component
Efficiency
Authorized in Mexico
Pfizer/BioNTech
United States / Germany
2 doses
Messenger RNA
95%
Authorized in Mexico
modern
United States
2 doses
Messenger RNA
94.5%
Authorized in Mexico
abdullah
Cuba
3 doses
recombinant protein
92.5%
Authorized in Mexico
Sputnik V
Gamaleya Research Institute
Russia
2 doses
Adenovirus 26 Adenovirus 5
91%
Authorized in Mexico
COVAXIN
National Institute of Virology Bharat Biotech
India
2 doses
Inactivated virus
81%
Authorized in Mexico
Synopharm
Beijing Institute of Biological Products
China
2 doses
Inactivated virus
79%
Authorized in Mexico
AstraZeneca / University of Oxford
Sweden - England
2 doses
Adenovirus ChAdOx1
76%
Authorized in Mexico
CanSinoBIO
Beijing Biotechnology Institute
China
1 dose
Adenovirus 5
64%
Authorized in Mexico
Johnson & Johnson
United States
1 dose
Adenovirus 26
67%
Authorized in Mexico
Sinovac
Sinovac Biotech
China
2 doses
Inactivated virus
50.38%
Authorized in Mexico
Novavax
United States
2 doses
recombinant protein
90%
Not authorized in Mexico
Pfizer/BioNTech
Country: United States / Germany
Dose: 2
Component: Messenger RNA
Effectiveness: 95%
Authorized in Mexico
modern
Country: United States
Dose: 2
Component: Messenger RNA
Effectiveness: 94.5%
Authorized in Mexico
abdullah
Cuba
3 doses
recombinant protein
92.5%
Authorized in Mexico
Sputnik V
Gamaleya Research Institute
Country: Russia
Dose: 2
Component: Adenovirus 26 | Adenovirus 5
Effectiveness: 91%
Authorized in Mexico
COVAXIN
National Institute of Virology Bharat Biotech
Country: India
Dose: 2
Component: Inactivated virus
Effectiveness: 81%
Authorized in Mexico
Synopharm
Beijing Institute of Biological Products
Country: China
Dose: 2
Component: Inactivated virus
Effectiveness: 79%
Authorized in Mexico
AstraZeneca / University of Oxford
Country: Sweden - England
Dose: 2
Component: Adenovirus ChAdOx1
Effectiveness: 76%
Authorized in Mexico
CanSinoBIO
Beijing Biotechnology Institute
Country: China
Dose: 1
Component: Adenovirus 5
Effectiveness: 64%
Authorized in Mexico
Johnson & Johnson
Country: United States
Dose: 1
Component: Adenovirus 26
Effectiveness: 67%
Authorized in Mexico
Sinovac
Sinovac Biotech
Country: China
Dose: 1
Component: Inactivated virus
Effectiveness: 50.38%
Authorized in Mexico
Novavax
Country: United States
Dose: 2
Component: recombinant protein
Effectiveness: 90%
Not authorized in Mexico

1. Do all vaccines work the same?
There are many different types of vaccines currently developed or in development against SARS-CoV-2, the virus that causes COVID-19. The main mechanisms of action of vaccines are described below:
- Nucleic acids: Vaccines that carry one or more genes of the coronavirus to human cells to produce viral proteins that trigger the immune response.
- Viral Vectors: Vaccines containing viruses designed to carry coronavirus genes. Viral vector vaccines enter cells and cause cells to produce viral proteins that are expressed on the surface of cells, to generate antibodies.
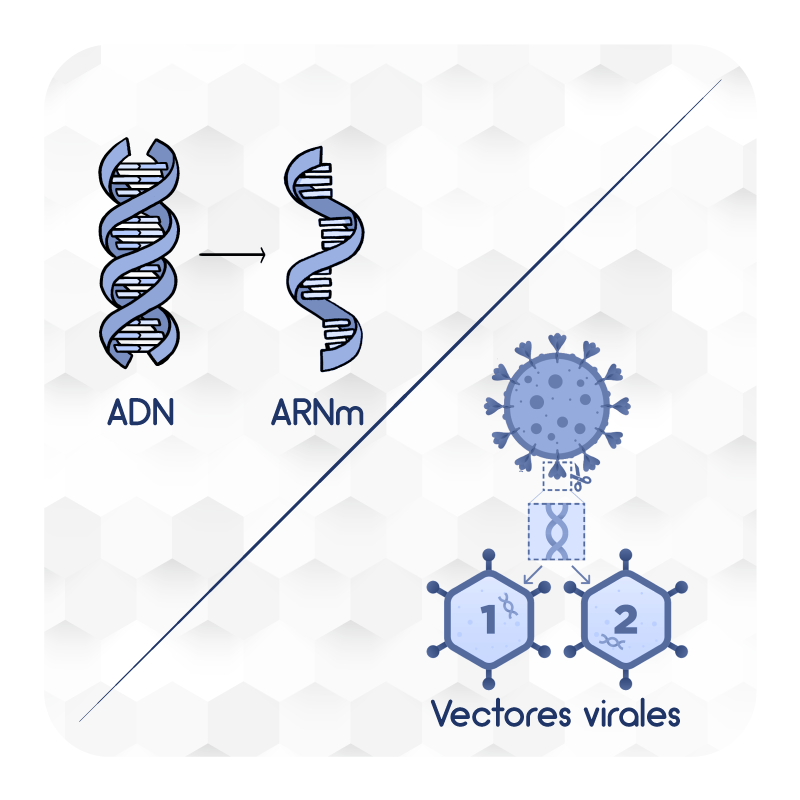
- Protein-based: Vaccines that contain complete proteins or protein fragments of coronavirus, but no genetic material. By detecting viral proteins, the immune response is triggered and antibodies and defense cells are generated.
- Inactivated viruses: Vaccines created from coronaviruses that have been inactivated with chemicals. These viruses are capable of triggering the immune response but not of causing the disease.
2. How do COVID vaccines work?
There are several types of vaccines currently developed or in development against SARS-CoV-2, the virus that causes COVID-19. Available vaccines include the following:
Nucleic acid vaccines
Both the Pfizer/BioNTech and modernvaccines use the messenger nucleic acid (mRNA), a molecule containing the instructions for protein production by cells. In the case of these vaccines, the mRNA has specific instructions to produce a viral protein called protein S, spike or spicule, which is essential for the virus to infect cells.
After vaccination, the cells receive the instructions and begin to manufacture the S protein and display it on their surface. The immune system then identifies the viral protein and triggers the immune response by producing antibodies and defense cells.
https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-vaccine/art-20484859
Viral vector vaccines
For AstraZeneca'svaccine, researchers added the gene that produces the S protein from the SARS-CoV-2 coronavirus to another virus called adenovirus. In this case, a modified version of a chimpanzee adenovirus known as ChAdOx1, which is harmless to humans, is used. This virus can enter cells, but cannot replicate inside them. Once inside the cell, the gene produces the viral protein which presents itself on the cell surface triggering the body's immune response.
https://www.nytimes.com/interactive/2020/health/oxford-astrazeneca-covid-19-vaccine.html
Like AstraZeneca'svaccine, the Russian Gamaleya Research Institute of Epidemiology and Microbiology's vaccine Sputnik Vand CanSinoBIO and Johnson & Johnson vaccine work via a viral vector. The vaccine uses two variants of adenovirus, a type of virus that causes colds, to which the gene with the instructions to produce the coronavirus spicule protein was included. The two types of adenovirus, Adenovirus 5 or Ad5 and Adenovirus 26 or Ad26 are designed to be able to invade cells to produce the spicule protein, but are not able to reproduce themselves. The strategy of using two types of adenovirus seeks to prevent the body's immune response from preventing the second dose of vaccine from working properly.
https://www.nytimes.com/interactive/2021/health/gamaleya-covid-19-vaccine.html
https://www.fda.gov/media/146305/download#page=2
Attenuated vaccine
Vaccines Sinovac Biotech, Synopharm and Covaxine vaccine are inactivated virus vaccines that use the "killed" version of the coronavirus that causes COVID-19.
Inactivated vaccines usually do not provide as strong protection as live vaccines. Several doses over time (booster vaccines) may be needed for continued immunity against the disease.
Recombinant protein vaccines
The Novavax is the first recombinant protein vaccine purified and assembled into nanoparticles. These types of vaccines are technologically designed to be very stable, easy to transport and store. They do not require a cold network at ultra-low temperatures, but rather the basic network of 2-8 degrees, which makes them ideal for massive application.
3. Do all vaccines give the same protection?
No. According to published research and experiments, after the second dose, or the single dose where appropriate, the efficacy of approved vaccines is as follows:
- Pfizer/BioNTechhas an efficiency of 95%
- modernof 94.5%
- SputnikV of 91.4%
- Novavax 90.4%
- Covaxine of 81% of
- Synopharm of 79%
- AstraZeneca's of 76%
- Johnson & Johnson (Janssen) of 67%
- CanSinoBio from 64%
- Sinovac from 50.38%
- Abdalac from 92%
https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html
https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-AZD1222-2021.1/
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/janssen.html
4. How do I know that a vaccine is safe?
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety.html
5. Are all vaccines safe?
Yes. Each vaccine producer publishes the results of their clinical trials where evidence of safety, efficacy, adverse effects and contraindications are presented based on the results of the experiments carried out.
This information, together with other details of the vaccine such as formulation and ability to generate protection, is submitted to the regulatory authorities to request authorization for use in each country. Authorization for use will then depend on the evidence that a vaccine is safe and effective in protecting the population against the disease.
In Mexico, COFEPRIS has already granted emergency use authorization to vaccines from Pfizer/BioNTech, AstraZeneca's, Sputnik V, Sinovac, CanSinoBIO, Covaxin, Johnson & Johnson, Moderna, Sinopharm and Abdala which means that these vaccines are allowed to be used only as part of the emergency health care by COVID-19.
https://www.bbc.com/mundo/noticias-55160530

1. Do all vaccines require two doses?
Vaccines of Pfizer, AstraZeneca's, modern, SputnikV, Sinovac and Sinopharm require two doses. Vaccines of CanSinoBIO and Johnson & Johnson They only require one dose.
Currently licensed COVID-19 vaccines require 2 doses for maximum protection:
- Pfizer/BioNTech: doses should be applied 3 weeks (21 days) apart
- modern: doses should be applied with an interval of 1 month (28 days)
The second dose should be given as close to the recommended interval of 3 weeks or 1 month as possible. However, there is no maximum interval between the first and second doses of either vaccine. The second dose should not be given before the recommended interval.
- AstraZeneca/Oxford: The two doses should be applied with an interval of 28 days.
- Sputnik V: The second dose should be applied 21 days after the first.
- Sinovac: The second dose should be given 2 weeks after the first.
- Covaxine: The second dose should be applied with an interval of 28 days.
- Synopharm: the second dose should be applied 3 weeks after the first.
- Novavax: the second dose is applied one month after the first.
- Abdullah: the second dose is applied 14 days after the first and the third 14 days after the second dose.
https://www.nytimes.com/interactive/2021/health/gamaleya-covid-19-vaccine.html
https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/janssen.html
2. Why is the application of complete schematics important?
Determining the volume and number of doses that people must receive to achieve the desired level of protection against disease is one of the main elements of the vaccine development process. This finally determines the vaccination scheme for each vaccine, which is submitted to the regulatory authorities in each country.
Therefore, to achieve protection against the disease, it is important to comply with the number of doses and the interval between each established by the producer and authorized by the regulatory authority.
https://www.fda.gov/news-events/press-announcements/fda-statement-following-authorized-dosing-schedules-covid-19-vaccines
3. What is the maximum time I have to administer the second dose of the vaccine?
According to the United States Centers for Disease Control (CDC), the maximum time to give the second dose of any vaccine is 6 weeks after the first dose is given.
- Pfizer/BioNTech: doses should be applied 3 weeks (21 days) apart
- modern: doses should be applied with an interval of 1 month (28 days)
- AstraZeneca/Oxford: the two doses should be applied with an interval of 1 month (28 days)
- Sputnik V: The second dose should be applied within 21 days of the first
- Sinovac: Doses should be applied with an interval of 2 weeks (14 days)
- Synopharm: Doses should be applied with an interval of 3 weeks (21 days)
- Covaxine: The second dose should be applied with an interval of 28 days.
- NOVAVAX: There is no information.
- abdullah There is no information
https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html
https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html
The Moderna COVID-19 (mRNA-1273) vaccine: what you need to know (who.int)
4. What happens if I only get one dose?
Failure to complete the recommended vaccination schedule may result in the expected level of immunological protection against the disease not being achieved. This is important because with some variants, such as Delta, the protective effect necessarily requires having the full regimen and 14 days having passed since the last application.
At this time, scientific evidence supports the schemes defined by producers and authorized by regulatory agencies such as COFEPRIS. Therefore, it is important that all people who receive their vaccines against COVID-19, have their complete schedules.
The Moderna COVID-19 (mRNA-1273) vaccine: what you need to know (who.int)
5. If I received the first dose with the Pfizer vaccine, can I have the second dose with another vaccine?
So far there is scientific evidence supporting the mixing of some schemes. However, there is no official recommendation in this regard at this stage of the vaccination strategy, since mixing schedules can impact the availability of sufficient doses for people who have not received any vaccine. Therefore, the current indication is that both doses of the scheme should be completed with the same product.
https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html
https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html
The Moderna COVID-19 (mRNA-1273) vaccine: what you need to know (who.int)
https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-AZD1222-2021.1/

1. After receiving the vaccine, how long am I protected?
For Pfizer, adequate levels of protection against the disease are achieved, in the 95% of those vaccinated, after two doses, 28 days after the first dose was applied.
The modern reaches adequate levels of protection of the 94.5% to the 14 days after the second dose.
Vaccine protection from AstraZeneca's, in its optimal scheme, occurs in the 76% of those vaccinated after two doses, between two and three weeks after application.
The Sputnik V, reaches an effectiveness of 73.1% from the first dose and 91.6% when applying the second dose (day 21).
For the company's CoronaVac vaccine Sinovac, an effectiveness of 50.6% is reached after the application of the second dose, 14 days after the first.
The Johnson & Johnson, reaches an effectiveness of 67% from day 14 of the application of the single dose.
The CanSinoBIO, reaches an effectiveness of 68,80% from day 28 of the application of the single dose.
Currently, the vaccine Covaxine no information is available about it.
The Synopharm it reaches its effectiveness 14 days after the second dose.
The Novavax it reaches its effectiveness 14 days after the second dose.
The abdullah it reaches its effectiveness 14 days after the third dose.
https://www.thelancet.com/action/showPdf?pii=S0140-6736%2820%2932661-1
https://www.thelancet.com/action/showPdf?pii=S0140-6736%2821%2900234-8
https://www.thelancet.com/action/showPdf?pii=S0140-6736%2820%2931605-6
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/janssen.html
2. If I have already been vaccinated, can I stop wearing face masks and can I stop social isolation?
No. Vaccines are not designed to block infection, but to decrease hospitalizations and deaths. Therefore, it is possible that vaccinated people can become infected, either symptomatically or asymptomatically, and thus be able to transmit the virus to other people. Even if you have received your complete scheme, the use of mouths must be maintained to prevent the spread of the virus.
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html
https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-vaccine/art-20484859

1. Can I get vaccinated against COVID at the same time I receive other vaccines?
While scientific evidence is currently limited, it does not appear that COVID-19 vaccines interfere with the immune response to other vaccines and vice versa. However, as long as you do not have other information, it is recommended wait 14 days after receiving the COVID-19 vaccine to receive another vaccine. Similarly, you must wait 14 days to receive the COVID-19 vaccine after receiving any other vaccine.
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html
https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-AZD1222-2021.1/

1. Can the COVID vaccine be applied to children and adolescents?
Approvals from regulatory authorities depend on evidence of safety and efficacy submitted by vaccine developers and producers. Currently, only one vaccine, Pfizer-BioNTech, has been authorized for application in the population aged 12 to 17 years in a 10-microgram presentation, and in girls and boys aged 5 to 11 years in a 3-microgram presentation.
Currently, clinical trials of several vaccines have already begun in the population between 6 months and 18 years of age, so it is expected that by the end of 2021 there will be sufficient evidence on the safety, effectiveness and practical aspects of vaccination of this population against the coronavirus. Later they will be authorized in younger populations, once there is confidence that the vaccine will not cause any serious adverse event in children.
https://www.thelancet.com/action/showPdf?pii=S0140-6736%2821%2900234-8


2. If I am pregnant, can I get the vaccine?
Vaccines are considered one of the safest medical products with the greatest benefit for people's health. Therefore, pregnant and lactating women can choose to apply the vaccine in a high-risk scenario. If you have questions about vaccination in pregnancy, it is advisable to consult a health professional.
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html
https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-AZD1222-2021.1/
3. If I am breastfeeding, can I get the vaccine?
While there is no evidence that COVID-19 vaccines can harm breastfed babies, there is not yet enough data to support widespread vaccination of breastfeeding mothers. However, lactating women who are part of a group that is recommended to receive the COVID-19 vaccine (for example, health personnel at high risk of exposure to the virus or with comorbidities) may choose to be vaccinated. If you have doubts about vaccination and breastfeeding, it is advisable to consult a health professional.
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html
https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-AZD1222-2021.1/
4. Can I get the AstraZeneca / Oxford University vaccine even if I am over 65 years old?
The vaccine developed by AstraZeneca/Universidad de Oxford It can be used by anyone over 18 years of age. Results from clinical trials have shown that the vaccine is safe and effective in adults over 65 years of age. It is very important that this age group is vaccinated against COVID-19 because they have a high risk of serious illness or death from the disease, situations that are prevented with the application of the vaccine.
https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-AZD1222-2021.1/
5. Can I get vaccinated if I am a person living with HIV?
According to the recommendations for the use of the vaccine AstraZeneca/Universidad de Oxford published by the World Health Organization, since this vaccine cannot be reproduced in the body, it can be administered to people living with HIV.
https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-AZD1222-2021.1/
6. Can I get vaccinated if I am a person who suffers from some type of alteration of immunity, whether due to treatment (for example immunosuppressants or chemotherapy) or disease?
According to the recommendations for the use of the vaccine AstraZeneca/Universidad de Oxford published by the World Health Organization, since this vaccine cannot be reproduced in the body, it can be administered to people who suffer from any alteration of immunity, immunodeficiency or immunosuppression.
https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-AZD1222-2021.1/
7. Can I get vaccinated if I am a person with an autoimmune disease (eg celiac disease, type 1 diabetes, rheumatoid arthritis, lupus erythematosus, multiple sclerosis)?
According to the recommendations for the use of the vaccine AstraZeneca/Universidad de Oxford published by the World Health Organization, since this vaccine cannot be reproduced in the body, it can be administered to people with autoimmune diseases.
https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-AZD1222-2021.1/

1. How long does the protection of the vaccine last?
It is not known how long the immunity conferred by a COVID-19 vaccine will last. This is because more data from ongoing and additional long-term studies is needed to understand how long protection lasts after vaccination.
2. Will we have to get vaccinated every year?
European Union authorities will coordinate independent studies on the use of COVID-19 vaccines in real life, to gather more information on their safety and long-term benefit in the general population. The results of these long-term studies will inform future vaccination strategies.

1. If I already got COVID, can I get the vaccine?
People who have already had COVID-19 or tested positive can benefit from the vaccine. There is currently not enough information available to say if people are protected against COVID-19 after they have had it (natural immunity) or for how long. Preliminary evidence suggests that the natural immunity may not last long, but more studies are needed to better understand this.
Other sources mention that due to the serious health risks associated with COVID-19 and the fact that reinfection is possible, it is advisable to get vaccinated. If you were treated for COVID-19 symptoms with monoclonal antibodies or convalescent plasma, you should wait 90 days to apply the corresponding vaccine. Talk to your doctor if you are not sure what treatments you received, or if you have more questions about getting vaccinated against COVID-19.
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/facts.html
According to data published by the World Health Organization, reinfection with SARS-CoV-2 is considered rare in the first 6 months after symptomatic infection. Therefore, while people who have suffered COVID-19 can safely receive the vaccine, it is recommended to wait 6 months after the illness to receive the first dose of the vaccine.
https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-AZD1222-2021.1/
2. How long after I got COVID can I get vaccinated?
The application of the vaccine should be postponed in people who have recently had COVID-19 and still continue with symptoms of this condition. Therefore, people with confirmed PCR disease and symptoms should wait for symptoms to disappear before receiving the first dose of the vaccine.
https://coronavirus.gob.mx/wp-content/uploads/2021/01/GuiaAplicacionVx_BNT162b_08Ene2021.pdf
https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-AZD1222-2021.1/
3. Which lasts longer, immunity after having COVID or the protection of vaccines against COVID?
The protection someone gets from having an infection (called “natural immunity”) varies by disease and varies from person to person. Because this virus is new, we don't know how long natural immunity might last. Current evidence suggests that contracting the virus again (reinfection) is rare within 90 days of the first infection with the virus that causes COVID-19.
We won't know how long immunity lasts after vaccination until we have more data on how well COVID-19 vaccines work under real-world conditions. Experts are working to learn more about natural immunity and vaccine-induced immunity.

Viruses are constantly changing through mutation as part of their natural evolution, and new variants of the virus are expected to appear over time. Sometimes new variants emerge and then disappear. At other times, new variants emerge and persist. Several variants of the virus causing COVID-19 have been documented worldwide during this pandemic.
The virus that causes COVID-19 is a type of coronavirus, a large family of viruses. Coronaviruses are so named because of the corona-like spikes found on their surface. These spikes are the structures by which the coronavirus causing COVID-19 binds to cells to infect them. Scientists monitor changes in the virus, including changes in the surface spikes. These studies, which include genetic analyses of the virus, help scientists understand how changes in the virus can affect how it spreads and what happens to people who become infected with it.
There are different variants of the virus causing COVID-19 circulating in the world, the most important of which are the following:
- The United Kingdom (UK) identified a variant called Alpha with a large number of mutations in the fall of 2020.
This variant spreads more easily and rapidly than the other variants. In January 2021, UK experts reported that this variant may be associated with an increased risk of death, relative to the other variants of the virus, but further research is needed to confirm this finding. Since then, it has been detected in many countries around the world. The first case of this variant in the United States was detected in late December 2020.
- Another variant called Beta appeared in South Africa, detected in October 2020, and shares some mutations with the Alpha variant. At the end of January 2021, cases caused by this variant were also reported in the United States.
- A variant called Gamma appeared in Brazil and was first identified in travelers from Brazil undergoing routine screening at an airport in Japan in early January. This variant contains a set of additional mutations that could affect its ability to be recognized by antibodies. The first case of this variant in the United States was detected in late January 2021.
- In India, in February 2021, the so-called Delta variant was identified, which is the most transmissible of all known variants and is currently the dominant variant in the world. This variant also has a certain capacity for immune evasion, which is why it has had an impact on the efficacy of all currently available vaccines. However, this impact is less in people who have completed vaccination schedules and who have had it for more than 14 days. filled.
- The Omicron variant was identified in South Africa in November 2021. It has 3 times more mutations in the spike protein compared to the Delta variant. It quickly spread across most continents and its mutations have been associated with immune evasion, both in vaccinated individuals and in previously infected people.

These variants appear to spread more easily and rapidly than the other variants, which could lead to more cases of COVID-19.
So far, studies suggest that antibodies generated through vaccination with currently licensed vaccines recognize these variants. This aspect is being carefully studied and further research is ongoing.
https://espanol.cdc.gov/coronavirus/2019-ncov/transmission/variant.html