La Administración de Alimentos y Medicamentos de Estados Unidos (FDA) otorgó la autorización a dispositivo Fitbit de Google, para utilizar su algoritmo de fotopletismografía que identifica la fibrilación auricular o ritmo cardiaco irregular.
The FDA ha autorizado el algorithm de Fitbit, que permitirá realizar un constant heart rate monitoring, ya que antes el wearable solo realizada a medición si el usuario lo indicaba de forma manual. En este sentido el dispositivo ahora realizará monitoreos constantes y notificará al usuario sobre ritmo cardiaco irregular y si hay riesgo de fibrilación auricular.
La fibrilación auricular, es un padecimiento difícil de detectar ya que los síntomas varían en cada persona y algunas veces puede no haberlos. A nivel mundial, esta condición afecta a más de 33 millones de personas, quienes tienen cinco veces mayor riesgo de sufrir un ataque al corazón.
The algorithm de fotopletismografía para la detección de fibrilación auricular (PPG Habib, en inglés) cuenta con validación clínica y funciona de la siguiente forma:
- Cuando el corazón late, el cuerpo se expande y se contrae basado en los cambios del volumen de la sangre.
- El sensor opción para el monitoreo del ritmo cardiaco de Fitbit puede detectar esos movimientos desde la muñeca.
- De esta forma puede medir el ritmo cardiaco de forma constante mientras el algorithm analiza signos potenciales de ritmo cardiaco irregular o fibrilación atrial.
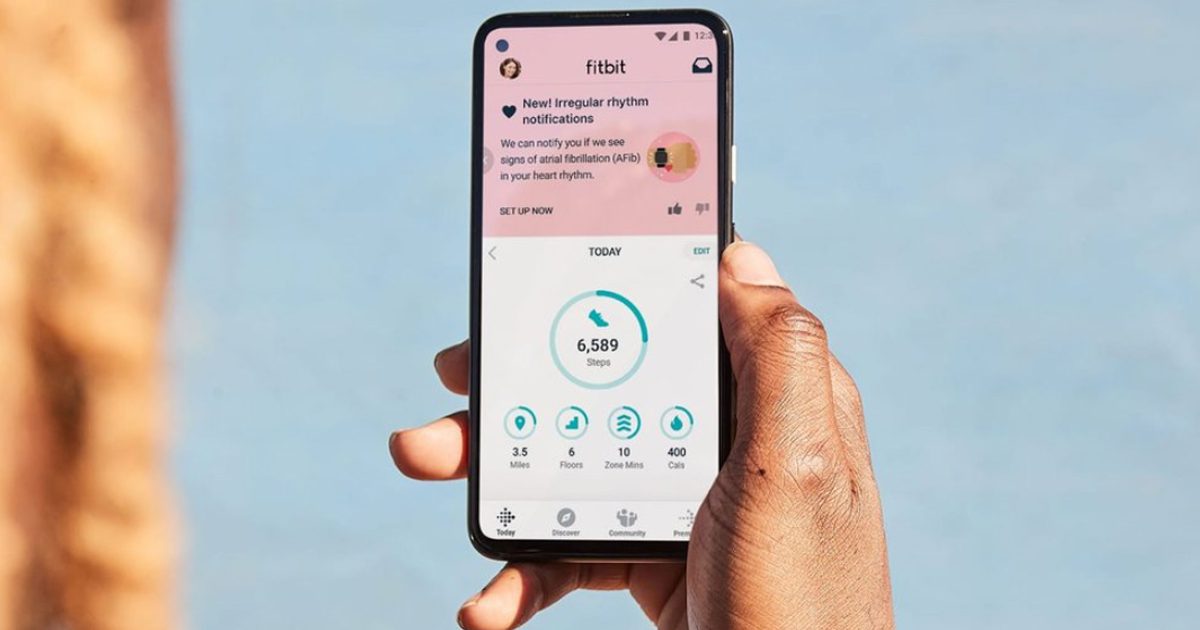
“Debido a que la AFib puede ser tan esporádica, la forma óptima de detectarla es a través de la tecnología de seguimiento de la frecuencia cardíaca cuando el cuerpo está quieto o en reposo, lo que hace que la detección durante la noche cuando las personas duermen sea especialmente importante”, explicó Google en su blog.
De esta forma, Fitbit puede monitorear la frecuencia cardiaca durante todo el día y alertar al usuario en caso de encontrar potenciales afectaciones. “Queremos que la detección de AFib sea lo más accesible posible para ayudar a reducir el riesgo de eventos potencialmente mortales, como un accidente cerebrovascular, y, en última instancia, mejorar la salud cardíaca general para todos”, concluye Google en su comunicado.







