The Food and Drug Administration (FDA), appointed Wysa, software of Artificial Intelligence (AI) and mental health, as an innovative device.
Wysa is a company specialized in mental health support through techniques of AI conversational. It recently received a groundbreaking designation from the FDA in its services of Digital Health and mental health, specifically involving patients diagnosed with chronic musculoskeletal pain, depression, and anxiety.
The Breakthrough Device Program of the FDA It is crucial to accelerate the development and approval of new medical devices and products that provide support in the diagnosis and treatment of life-threatening diseases.
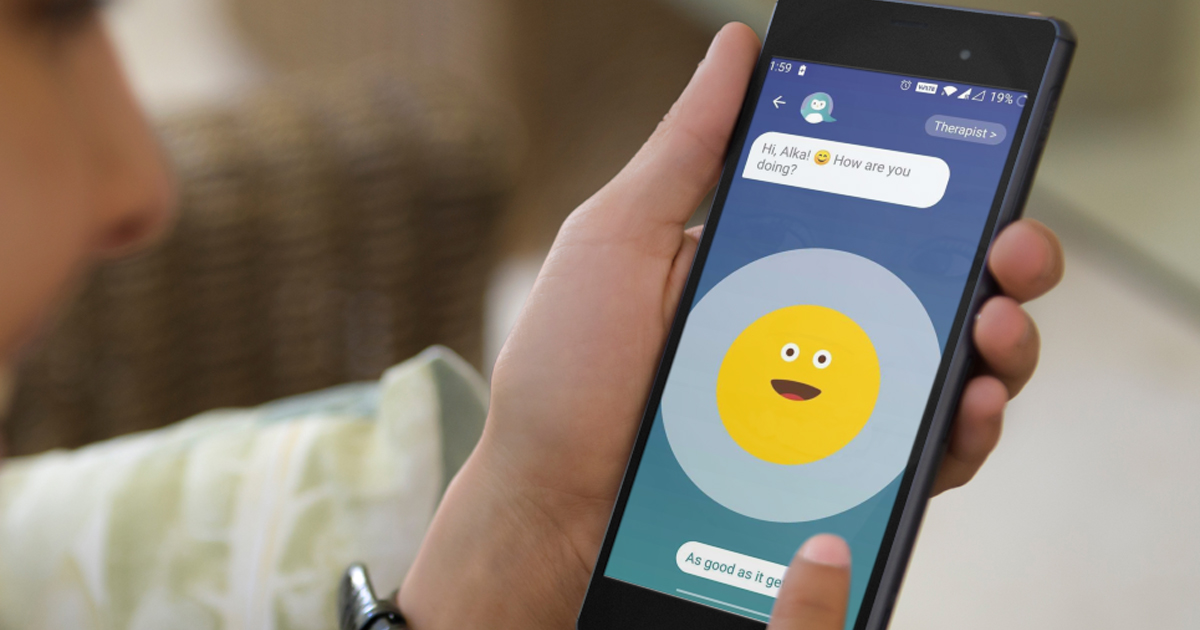
Wysa's bot has been trained to deliver cognitive behavioral therapy, which is delivered via smartphone. In addition, it has been shown to reduce symptoms of depression and anxiety, as well as improve physical function in patients with musculoskeletal conditions, as shown in a independent study published in The Journal of Medical Internet Research (JMIR).
The study aimed to compare changes in participants' mental health over two months. Results showed that patients who received digital mental health interventions as part of orthopedic care achieved improvements in pain, physical function, and depression.
“We are delighted to achieve this significant designation of the FDA and we look forward to working closely with the Agency to continue the development of cognitive behavioral therapy based on the AI"We're always looking for a solution to this problem," explained Jo Aggarwal, CEO and co-founder of Wysa. He also explained that the company's mission is to provide diverse mental health options and do so with a platform that's always available.