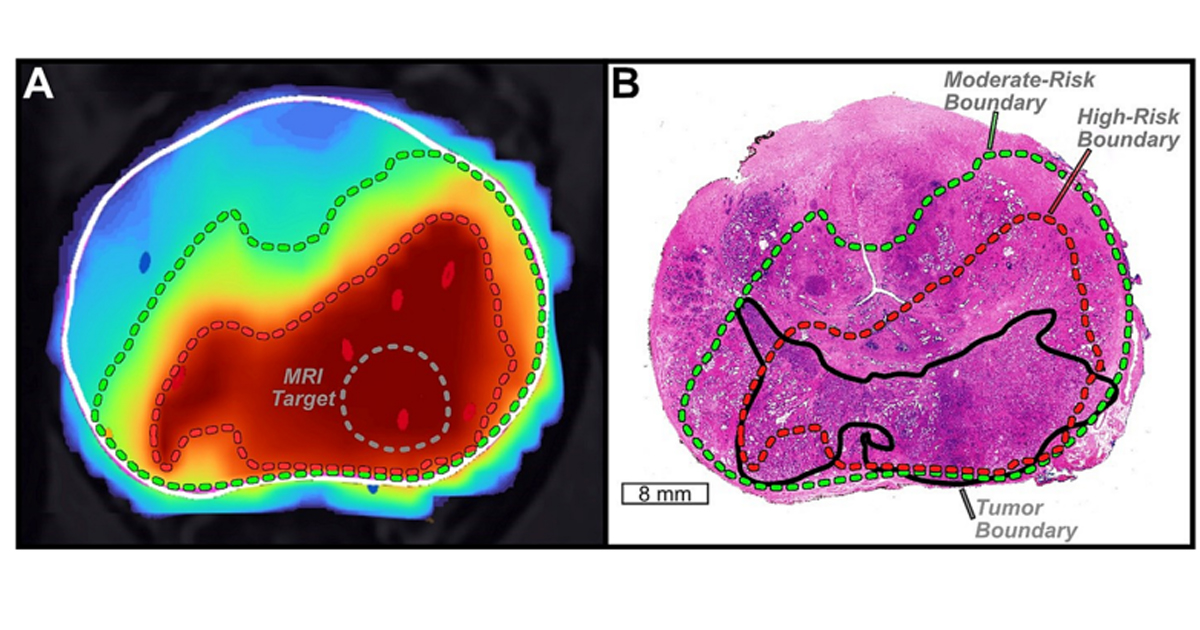
"¿Qué se requiere para llevar la innovación transformadora a los pacientes?", inicia el estudio conducido por la Universidad de California en Los Ángeles (UCLA).
El UCLA Biodesign Hub for MedTech and Digital Health es una red regional que promueve el progreso y avance tecnológico de tecnologías de digital health con potencial de comercialización.

La Iniciativa de UCLA fue la creación de un estudio sobre toda la industria de medicina y digital health, para conocer costos y tiempos de aprobación y regulación de avances tecnológicos en medicina. El estudio esta respaldado por la Administración de Desarrollo Económico (EDA, por sus siglas en inglés).
A través de www.medtechstudy.com UCLA busca que compañías de Digital Health, expertos, ejecutivos de tecnología médica, y líderes de opinión en esta tecnología, participen en su estudio sobre el impacto de la regulación rembolso de 2010 a 2020 a través de entrevistas de 30 minutos.
El participante debe elegir un dispositivo médico o de digital health aprobado en Estados Unidos por la FDA entre 2010 y 2020.
The interviews include the following questions:
- ¿Cuánto tardan realmente las innovaciones en llegar al mercado?
- ¿La digital health and Artificial Intelligence/Aprendizaje Automático tienen un camino claro hacia el mercado?
- ¿La designación de avance está rompiendo las barreras regulatorias de acceso?
- ¿Es el reembolso la nueva barrera a la innovación?
- ¿Estados Unidos sigue el ritmo de Europa, Japón y China?
- ¿Qué sigue en el horizonte regulatorio y de reembolso?