According to information published in the scientific journal Cell, this would be possible through the detection of the enzyme Cas13 which, in its binding to the RNA of the SARS-CoV-2 virus, produces fluorescence that can be detected by the camera.
Scientists and researchers from various institutes and departments, from universities in California such as the University of California, San Francisco and the University of California, Berkeley, as well as from the Chan Zuckerberg Biohub and the J. David Gladstone Institutes, have developed a test for the detection of SARS-CoV-2 through RNA obtained through nasal tests whose results could be analyzed through a microscope adapted to a cell phone.
The first results of this innovation will be published in the scientific journal Cell in its January 2021 issue, under the title: Unamplified detection of SARS-CoV-2 with CRISPR-Cas13a and cell phone microscope. Daniel Fletcher, a bioengineer at the University of California at Berkeley and co-author of the paper, explained: “Our study shows that we can do the detection part of this assay very quickly, making the measurement with mass-produced consumer electronics.”
In addition, he emphasized that very sophisticated laboratory equipment is not required to achieve the results they seek. “We chose to use mobile phones as the basis for our detection device since they have intuitive user interfaces and highly sensitive cameras that we can use to detect fluorescence,” he explained.
The research proposes an alternative to PCR tests, which are the most reliable tests for detecting the presence of the virus in the body. These tests require that the virus RNA is first converted into DNA, a two-step chemical reaction that considers amplification to obtain enough DNA to make detection possible.
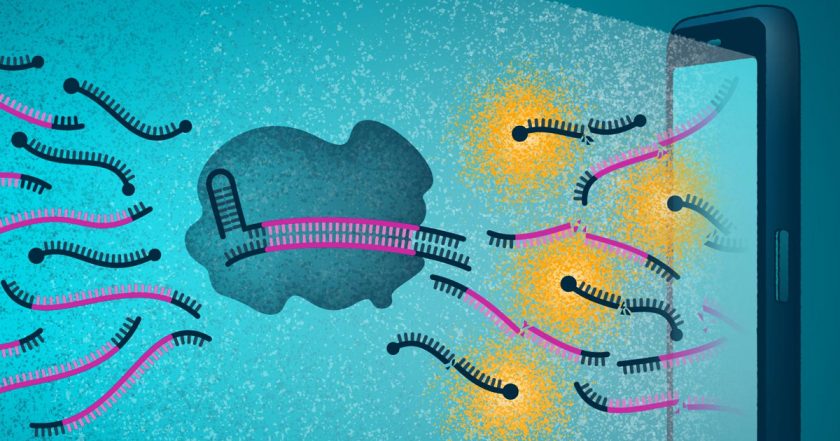
The proposed research trials are based on CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats), a family of DNA sequences, which are found in the genomes of organisms, and Cas13a (also known as C2c2) a class 2 type VI protein related to RNA. “One reason we’re excited about CRISPR-based diagnostics is the potential for quick, accurate results at the point of need,” explained Jennifer Doudna main researcher at the J. David Gladstone Institutes.
“What really makes this test unique is that it uses a one-step reaction to directly test the viral RNA, as opposed to the two-step process in traditional PCR tests,” explained Melanie Ott, who serves as director of virology at Gladstone, “The simpler chemistry, paired with the smartphone camera, cuts down detection time and doesn’t require complex lab equipment. It also allows the test to yield quantitative measurements rather than simply a positive or negative result,” she concluded.
The goal of the researchers is to make the device and the tests sufficiently reliable for application and implementation. And thus, offer tests for a faster diagnosis of the presence of virus in the body, because the waiting time to know the results would be 15 to 30 minutes. Beyond the speed of the test, it is also an opportunity to solve one of the biggest problems during the pandemic, the availability of mass testing.
In addition to knowing the positive result of SARS-CoV-2, the test seeks to know the viral load of the virus in order to later predict the evolution of the disease. “When coupled with repeated testing, measuring viral load could help determine whether an infection is increasing or decreasing,” Fletcher explained.
You can read the article published in Cell, in the following link: https://www.cell.com/cell/pdf/S0092-8674(20)31623-8.pdf.