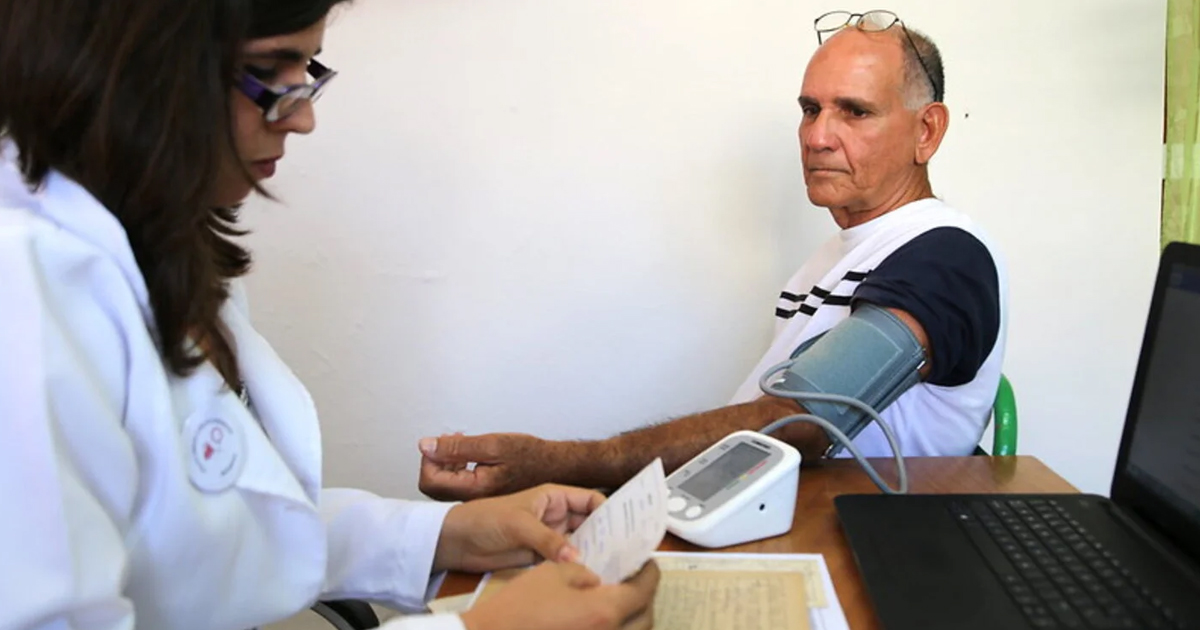
Thanks to the Precertification Program that is responsible for regulating food and medicine through software within the US government, several companies obtained the registration of medical devices, algorithms based on artificial intelligence and diagnostic tools.
The Food and Drug Administration Agency (FDA) has been focusing efforts on adopting innovations. Dr. Norman Sharpless, the agency's new commissioner, has pushed the decision to call on new companies to test the current framework of their Digital Health Software Precertification Program.
The objective of the program is to facilitate the regulation of Digital Health products prone to frequent updates. In this way, new companies and startups can ensure that their efforts are 100% certified by the United States Government and thus be exempt from fines and regulatory notices.
The Center for Devices and Radiological Health of the FDA (responsible for giving continuity to the program) has only one priority: patients. Providing access to high-quality, safe and effective innovations, from mobile applications, sensors for measuring physical activity, among others, is paramount. In the same way, the use of algorithms embedded in digital platforms that favor the clinical decision making that doctors make daily, is of vital importance.
The conscious effort of the United States government to improve the user experience through Digital Health doesn’t stop at the certification of new companies. They have also focused on alerting patients and health service providers, by warning them about the dangers of devices and software for the control of diabetes and other chronic diseases that have not been evaluated by the agency.
“We must ensure that we can continue to provide a first standard in terms of safety and effectiveness. We believe that the agency's guidance will help advance the development of these innovative products," said Dr. Scott Gottlieb, former FDA commissioner.
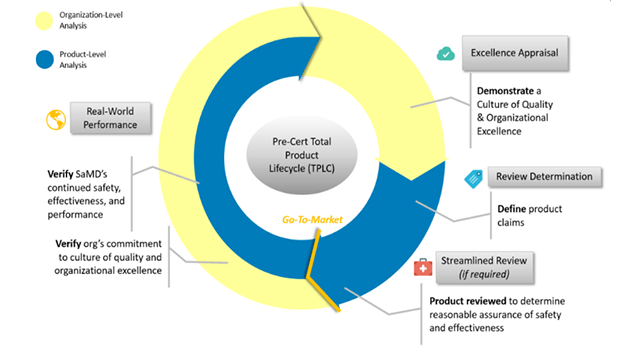
The FDA's total product life cycle approach allows you to evaluate and monitor a software product from its pre-marketing development to post-sale performance. This brings a specific benefit, which is a personalized and pragmatic regulatory oversight that evaluates organizations of all sizes, to establish the confidence that they have a culture of quality and excellence that results in safe products for the patient.
It’s very important to know, publicize and verify that all devices and technologies adopted for health care comply with quality and protection standards in favor of users.