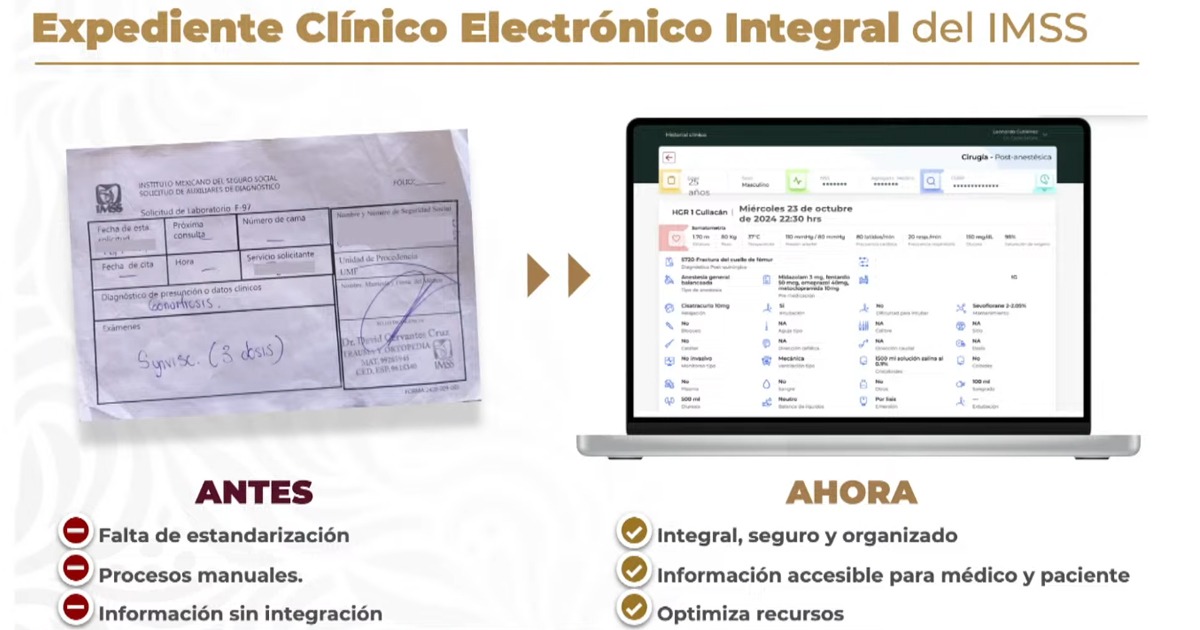
The University of Oxford created a digital platform aimed at personnel working in the area of medical devices and their development, to learn about and understand the regulations of various devices.
The University of Oxford, through its Natural Interaction Laboratory (NIL) in the department of Engineering Science, has created a web-based digital tool aimed at people working around medical device development. The tool was funded by the Engineering and Physical Sciences Research Council (EPSRC) of the United Kingdom and the European Institute of Innovation and Technology (EIT), a European Union (EU) institution.
Oxford Global Guidance is a digital tool to facilitate and streamline understanding of how medical device regulations apply to new products or products under development. On their website, they indicate that the tool was launched earlier than expected, as due to the COVID-19 pandemic, they sought to drive a functional platform for medical equipment personnel to navigate and learn about medical device regulations and requirements, specifically in the EU.

The platform is divided in four steps, first the online support for the description of the device; subsequently, the platform presents a questionnaire to evaluate whether or not it is a medical device according to EU regulations; the third step, the classification involves another questionnaire to find out to which classification the device belongs according to EU MDR and IVDR regulations; finally the result, will provide a more extensive overview for decision making with experts in healthcare institutions or for the improvement of the device in its development process.
This process takes at least 20 minutes and is offered free of charge through a basic registration via an email address. It is currently in a process of continuous improvement, to add new features and better navigation.